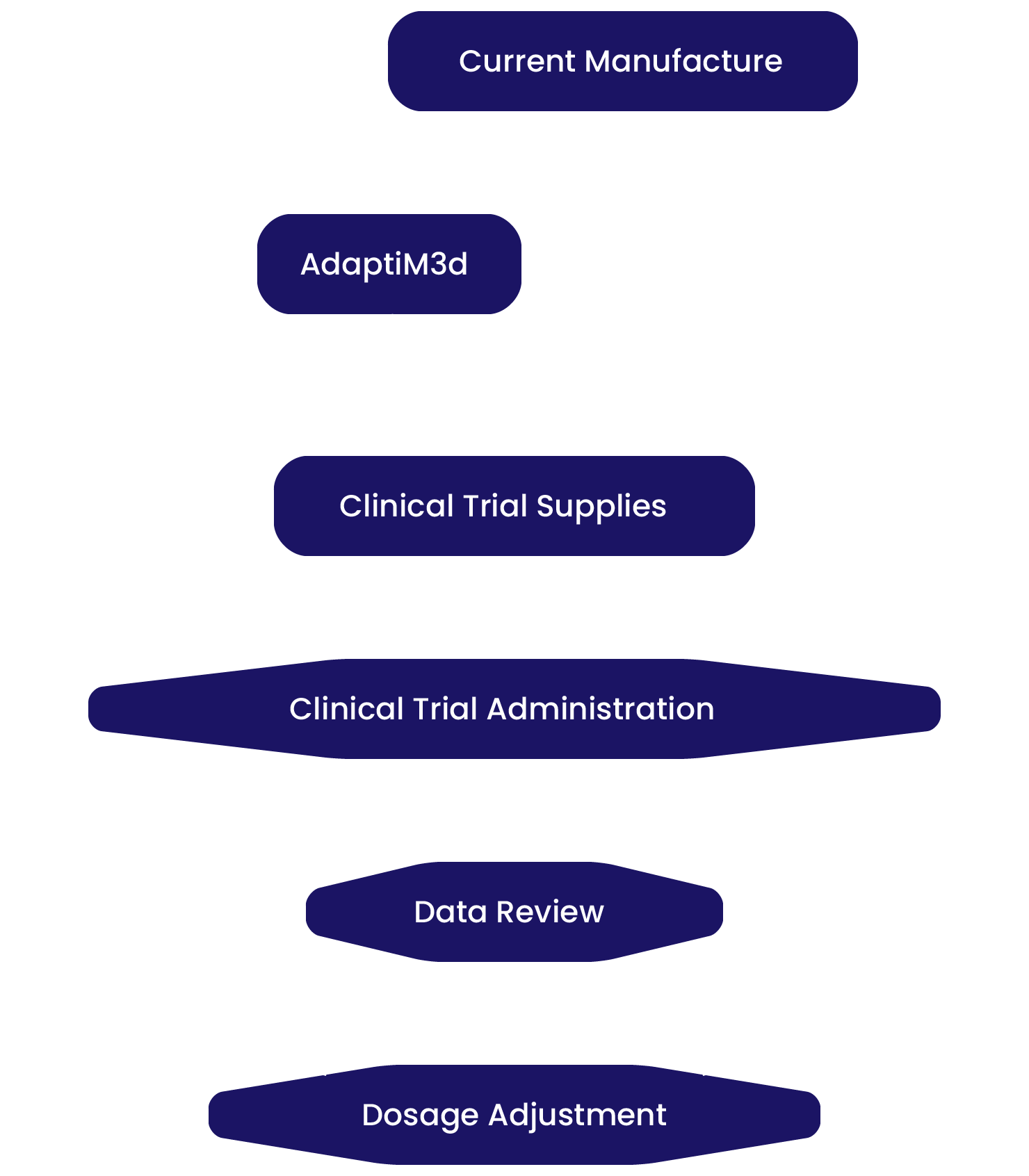
The AdaptiM3D Solution
AdaptiM3D's approach substantially eliminates the 6-month delay for reformulation and manufacturing.
By replacing multiple processes with a single operation, we save at least 2-3 months on the critical path to product development.
Benefits for Small Companies
For small biotech companies, time and cost savings are particularly crucial. Our technology allows these early adopters to be more nimble in their clinical development, adjusting doses quickly without the traditional delays and costs associated with reformulation.
One third of the 500,000 annual global early phase clinical trials are halted due to prediction errors of the required human dose, with the initial formulation being found to be unsuitable, causing the trial to be stopped while a new dose of the product is reformulated and manufactured. Manufacturing using existing processes requires the use of several steps (e.g. blending, granulation, drying, tabletting and coating), and process conditions have to be balanced among these operations to produce a dose form that delivers the drug in the patient’s body.
Due to the interactions among these process steps and the need to balance many formulation and process aspects to produce a dose form that works in the patient, reformulation takes 3-6 months and is on the critical development path for product launch. This reduces the on-patent sales life (and for a large pharmaceutical company the value of this would a loss of many millions of pounds) and is a fundamental problem affecting the industry.